Πληροφορίες προϊόντος
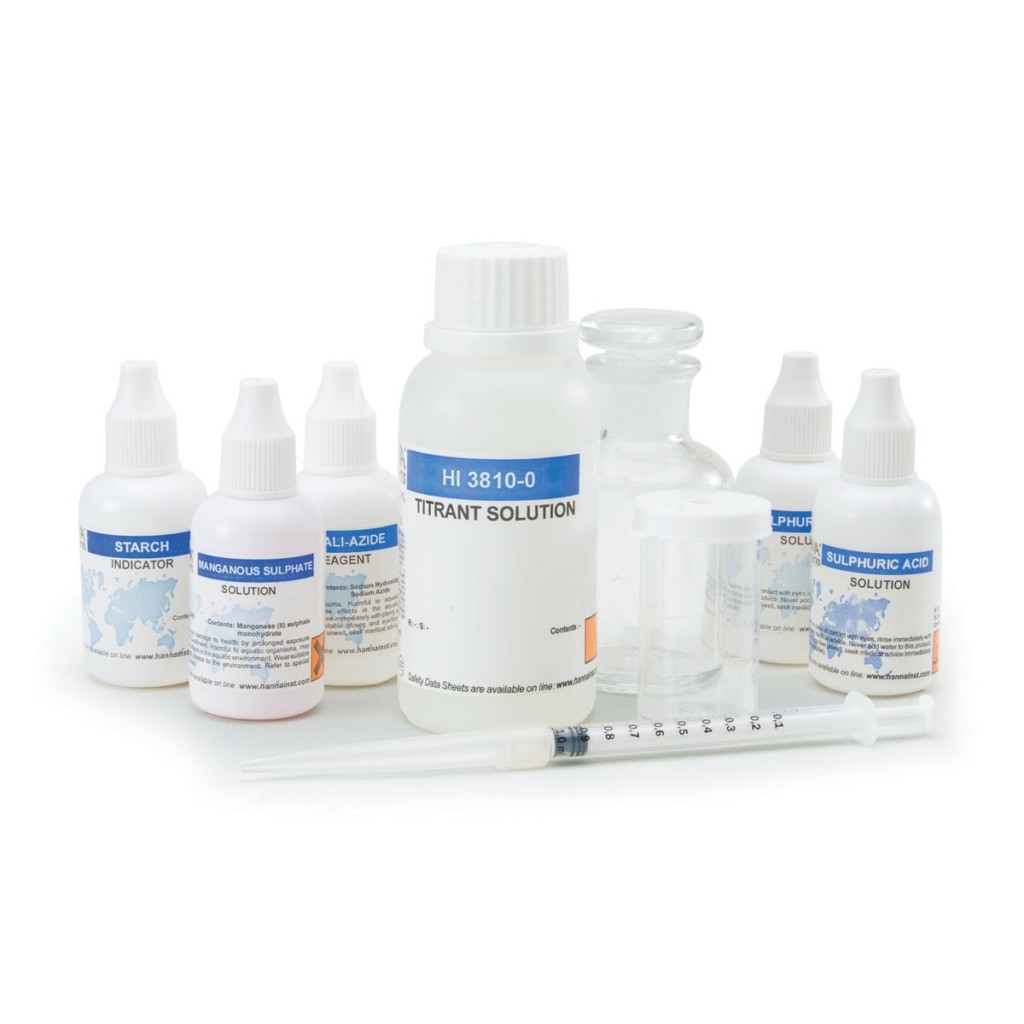
The HI3810 is a chemical test kit that measures dissolved oxygen by modified Winkler titration. The HI3810 is supplied complete with all of the reagents and equipment necessary to perform approximately 110 tests.
Pre-made reagents for ease of use
All reagents marked with expiration date and lot number for traceability
Manual titration performed with starch indicator
Details
The HI3810 is a titration-based chemical test kit that determines the dissolved oxygen concentration within the 0 to 10 mg/L Ol2 range. The HI3810 is supplied with all of the necessary reagents and equipment to perform the analysis. The test kit contains enough reagents for perform approximately 110 tests.
Features at-a-glance
- Complete setup
- All required materials are included with the test kit, such as the glass stoppered bottle, indicator and reagent bottles, and calibrated syringe.
- High resolution
- Readings from 0 to 10 mg/L are determined to 0.1 mg/L resolution.
- Replacement reagents available
- There is no need to buy a new kit when reagents are exhausted. The HI3810-100 can be ordered to replace the reagents supplied with the kit.
Significance of Use
The concentration of dissolved oxygen in water is extremely important in nature as well in man’s environment. In oceans, lakes, rivers, and other surface water bodies, dissolved oxygen is essential to the growth and development of aquatic life. Without oxygen, water can become toxic due to the anaerobic decaying of organic matter. In man’s environment, water must contain at least 2 mg/L of oxygen to protect water pipes from corrosion. However, boiler system water, in many cases, cannot contain greater than 10 mg/L oxygen.
A modified Winkler method is used in the HI3810 test kit. Manganous ions react with oxygen in the presence of potassium hydroxide to form a manganese oxide precipitate (Step 1). An azide is present to prevent any nitrite ions from interfering with the test. With addition of acid, manganese oxide hydroxide oxidizes the iodide to iodine (Step 2). Since the amount of iodine generated is equivalent to the oxygen in the sample, the concentration of iodine is calculated by titration of thiosulfate ions that reduce the iodine back to iodide ions (Step 3).
Step 1: 2Mn2+ + O2 + 4OH– → 2MnO(OH)2
Step 2: MnO(OH)2 + 2I– + 4H+ → Mn2+ + I2 + 3H2O
Step 3: I2 + 2S2O32– + S4O62-